New Product Introduction
We share your commitment to excellence in NPI program execution
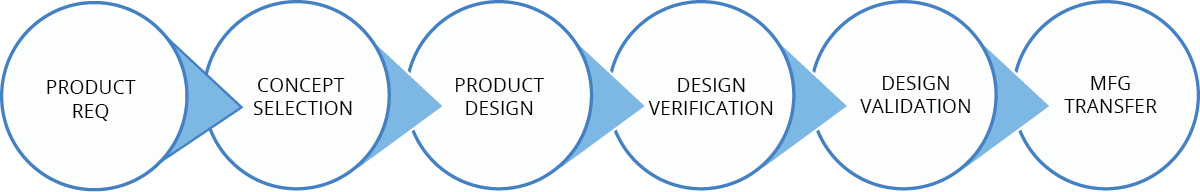
Your optimal New Product Introduction outcome requires application of experience, expertise, and program management disciplines in executing complex and critically linked steps throughout the effort. JunoPacific’s New Product Introduction (NPI) team utilizes state-of-the-art equipment and analytical tools in combination with extensive medical device development expertise. Our singular focus is ensuring your successful NPI program.